|
 |
 |
 |
|
Chemical Education International, Vol. 7, No. 1, AN-1, Received October 21, 2006 To the Youth of the World Who Aspire to a Career in Chemistry Message
from Nobel Laureates to Young People (5) INTRODUCTION The intended readership of the interviews published in CEI are senior high school students who are at a point in their life where they must make decisions about their future career, or first year university students in science and technology who must begin to specialize in a chosen field of study. We are extremely grateful to Prof. Ryoji Noyori* for his appreciation of the idea of this series of interviews and for kindly sparing us his precious time. This interview with Prof. Ryoji Noyori (left, on picture 1 above), by Prof. Yoshito Takeuchi and Prof. Masato M. Ito was carried out at the office of the President, RIKEN on March 16, 2005. *The 2001 Nobel Prize in Chemistry was shared jointly by Prof. Ryoji Noyori (Nagoya University), Dr. William S. Knowles (Monsanto Co.) and Prof. K. Barry Sharpless (Scripps Research Institute) by virtue of their achievement in catalytic asymmetric synthesis. CEI:
Chemical Education International RN: Professor Ryoji Noyori CEI: First, let me ask your background. Were there any special circumstances or a particular stimulus that led you to pursue a career in science? RN:
I was born in 1938 and I entered primary school in the year the
Second World War ended. This period corresponds to a time in Japanese
history when the country was in an economically difficult situation,
and also in a state of confusion. This might have made a difference
between me and Japanese children of my era with children who grew
up in other countries. I suppose my desire to become a scientist
was fostered in such an atmosphere. CEI: I belong to much the same generation as yours. I also remember the impact of the news very vividly. RN:
Just after I was born, my father, accompanied by my mother, went
to Europe to inspect research facilities there. He was lucky in
that one of the passengers on the ship, the Yasukuni-maru, was a
young Prof. Yukawa who was also going to Europe to attend the Solvey
Congress. For one month my parents traveled on the ship and participated
in dance parties and mahjong games. My father told me that Prof.
Yukawa was asked to deliver a public lecture which turned out to
be easy to follow though the topic was very difficult.
Photo 2
This photo was taken in Hawaii in 1939. The Kamakura-maru stopped
at Hawaii on her way from San Francisco to Yokohama. Prof. Yukawa
(2nd from left; 32 years old at that time), Mr. Kaneki Noyori (3rd;
28 years old) and Mrs. Suzuko Noyori (24 years old). CEI: Tell us about your primary school life. RN:
My primary school was attached to the Department of Education, Kobe
University, and located at the foot of Mt. Rokko, which is rich
in nature. Our school was a kind of experimental school, and there
were many splendid teachers who taught us with care. I remember
there was no particular subject which I was fond of, but I could
say that I enjoyed studying in general. CEI: You entered Nada Middle School and then Nada High School, which is one of the leading secondary education schools in Japan. What were your experiences like there? RN:
I remember it was during the spring break before I entered middle
school when an event took place which influenced my future career
very significantly. I call the event "the Nylon case".
For some reason or other, my father took me to the announcement
of the new product, nylon, by the Toyo Rayon Co. (now TORAY). I
attended to the meeting as a sole child among many adults. The President
of the company told the audience something to the effect that the
amylan (nylon) could be prepared from coal, water and air. I was
very impressed, knowing that expensive materials such as nylon could
be produced from very cheap raw materials. I was surprised to learn
the power of chemistry.
Photo 3 Prof. Noyori when he was a student at Nada High School. CEI: Judging from your story, we could say that you had pursued a career as a chemist from your middle school years straight through. RN:
This is so, though I am not sure which is better; to go straight
through or to take a broader, less direct path. As for me, I made
up my mind in my childhood to become a chemist. CEI: Please tell us about school life at Nada High School. RN:
The Kobe First Middle School (now Kobe High School) was famous for
its severe discipline. It was said that pupils had to eat their
lunch, without sitting on their stool, while other pupils on duty
cleaned the classroom. On rainy days, one pupil ate his lunch while
the other held an umbrella. After the war, excellent teachers from
Kobe First Middle School moved to Nada Middle and High Schools mostly
because of changes made to the educational system.
RN:
I was attracted by the reputation of Prof. Ichiro Sakurada and this
was the reason why I entered Kyoto University. In the first two
years of general education, I must admit I was not very diligent.
I joined the rugby football club though I was not a regular member.
Drinking alcohol and having fun with friends were my main activity
during this period. CEI: You then began to investigate your main theme, asymmetric synthesis. RN: A turning point in my career as a chemist was my appointment as an assistant, a junior faculty member. I intended to continue my study to obtain a higher degree after I finished my research for the MS degree. Then Professor Nozaki was promoted to full professor and intended to organize a new research group. Prof. Nozaki wanted me to be his assistant instead of continuing on with graduate study. My original intention was to obtain a PhD and then to enter the chemical industry as a senior industrial chemist. I was, however, persuaded by him, and finally accepted his offer.
Photo
4
At Kyoto University with young students. The
theme of my research was the study of carbenes, which are short-lived
reaction intermediates. Carbenes, which are generated by thermolysis
or photolysis of diazoalkanes, can exist in triplet or singlet forms
with the reaction proceeding non-selectively.
Two nights' work was necessary to obtain enough sample to measure its optical rotation. Though the work was tough, I vividly remember the elation I felt when we found that the expected results were obtained.
RN:
Not at all. This experiment was aimed to prove the existence of
the complex between carbene and copper ion. I hadn't yet thought
of asymmetric synthesis at that time. CEI: In a word, it was an attempt to challenge nature, was it? RN: Indeed, exactly so. What I aimed at was not "catalysts as they are", but "catalysts as we want them to be." We believed that it should, in principle, be possible to design and synthesize any compound. My idea was to incorporate appropriate electronic and steric effects into molecules and to use them as catalysts. This was in 1966. Around that time, although some homogeneous catalysts such as metal carbonyls were known, the notion of molecular catalysts which would make use of the characteristics of molecules was not yet developed. CEI: How you could succeed in the challenge to Pasteur? RN:
After this I moved to Nagoya University, but there were campus troubles
at that time and I was allowed to study abroad at Harvard University
with Prof. E. J. Corey*1 as my supervisor during this
period. There I encountered hydrogenation reactions.
(Asymmetric synthesis is a synthetic reaction in which unequal amounts of (+)- and (-)-enantiomers are formed. If one enantiomer is formed in a 100 % yield then the reaction is called perfectly enantioselective. See reaction schema)
Photo
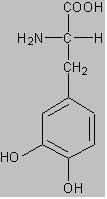
5 The Nobel Prize diploma awarded to Prof. Noyori. I returned to Japan and was so involved in so many things that it was difficult for me to start research on asymmetric synthesis. Prof. H. B. Kagan and Dr. Knowles advanced their research into asymmetric hydrogenation and Monsanto Co. successfully developed the industrial synthesis of L-DOPA. (DOPA = 3,4-dihydroxyphenylalanine)
Fig. 2 The structure of DOPA. L-DOPA is the left-handed enantiomer. I
was unable, however, to even start my research along these lines.
Many chemists thought that there was not much left to be investigated
in the field of asymmetric hydrogenation, but it turned out in contrast
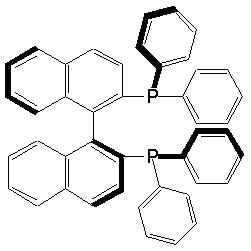
that the study had only just begun. CEI: Indeed. We can say that splendid functions come from beautiful structures. RN: I believe so. The dictum of the "Bauhaus" movement of Germany tells the truth. "The beauty of that molecule is brilliant". I started research on this molecule from 1974. We encountered a series of difficulties, and many Japanese and foreign chemists withdrew from such study. In 1980, we finally managed to publish a paper on the asymmetric synthesis of amino acids based on the BINAP chemistry. One of my coworkers on this project was the late Prof. Hidemasa Takaya who was at the Institute of Molecular Science and Kyoto University. Sadly, he passed away in 1994 while he was on a lecture tour in Germany.
Fig. 3 The structure of BINAP. Notably,
as we continued the study, it became clear that the complex of rhodium
(Rh) and BINAP as catalyst was the worst possible combination in
view of the reaction mechanism. The result looked nice since nearly
a 100 % enantiomeric excess was obtained. However, this result was
beside the point as Prof. J. Halpern pointed out. The hydrogenation
reaction proceeded via complexes between the BINAP-Rh catalyst and
an alkene substrate. Two equilibrating intermediates were formed;
one was the major, favored complex and the other a minor isomer.
The point is that the minor intermediate was more reactive and gave
the desired enantiomeric isomer, whereas the major complex was less
reactive to result in the wrong enantiomeric product. BINAP is very
selective, and only the main complex could be observed using NMR.
This complex was, however, not very reactive, and its minor isomer,
which was not detectable by NMR, was active. This meant that a very
strict control of the reaction condition was required to form the
necessary minor complex. How you could form in sufficient quantity
a compound which was hardly detectable? The suitable reaction condition
was obtainable only with a very dilute solution and under a reduced
pressure. Two years were necessary to find that appropriate reaction
condition. CEI: Was the competition hard? RN: Not necessarily. The essence of research is how to reply to the questions cast by chemistry. Hence I did not care at all about competition with other chemists. CEI: Anyway, you solved the problem successfully. How did you achieve success? RN:
There were two problems to be solved in asymmetric hydrogenation.
One was how to achieve high ratios of (+)-enantiomer to (-)-enantiomer
as close as possible to 100:0. This could be solved by designing
the shape of the catalyst. This success attracted considerable attention.
CEI:
What we have heard is really very valuable and useful for young
people. The way you carry out research seems to have been influenced
by the training you had received in Kyoto University concerning
your research on reactive intermediates. RN:
It was already discovered by Wilkinson and others that Ru as well
as Rh could cleave hydrogen molecules and might be suitable for
the hydrogenation catalyst. Famous chemists such as Kagan and Knowles
had already obtained excellent results using Rh catalysts. Hence
many chemists were tempted by these findings to study Rh.
CEI: Can you tell us about some lessons you have learned over your long research career that might be helpful to young people. RN:
After more than thirty years' studying hydrogenation, I realized
that "fact is the enemy of truth". This is the dialog
of Don Quixote in the musical "Man of La Mancha" written
by Dale Wasserman. Facts are valid only under limited conditions
while the truth is something general that is behind the facts. The
facts known when we initiated the study of hydrogenation were scientifically
correct at that time, but it was only a very small part of the world
of asymmetric hydrogenation. The truth about asymmetric hydrogenation
is very deep and expansive. CEI:
This is really the crucial point. We tend to be satisfied when we
feel we have identified the facts. It is difficult to go on further
from this point. RN:
The world of science will expand infinitely. However, scientific
research today tends to be too special and fragmental. What is needed
of science is 'generality'; that all fields are combined into one
universal science. So far scientists have not been particularly
eager for this. Scientists must have a wider view of nature. As
I have said before, scientists must make a line from a point, expand
to a plane from a line and then design and construct a space. Chemistry
is a science of matter and is the basis of modern civilization.
Generality is particularly important for chemistry. CEI: I am afraid that it is rather difficult, under the present educational system, for young people to grow up with such a wide perspective. Perhaps you have some advice for schoolteachers that might be useful. RN:
I expect teachers will understand the reason why human beings devote
time to science, and convey this point to their students. Scientists
devote themselves to science not because of bread, but because science
will bring them spiritual fulfillment.
My motto is "Research should be fresh, simple and clear." I would advise young people to consider the way to promote science in the right direction, and tackle problems as legitimate and fundamental as possible. CEI: The challenge to solve legitimate and fundamental problems is indeed a challenge to create a new field! RN:
From my forty years' experience as a researcher, I learned that
a research project has its own lifetime. In most cases this is from
twenty to thirty years. A new jump, making the results obtained
thus far as the foundation for further expansion, is required when
the project is fully grown.
When
you tackle with a new project, at first you will be in a minority
group. Originality tends to be a lonely existence. I hope young
people will not fear loneliness. Rather, I hope they will be proud
of it. There seems to be a kind of misunderstanding of the meaning
of democracy; thus it tends to be accepted that the majority is
mighty and correct while the minority is wrong. People tend to belong
to the majority because it is safer. Such a tendency is by all means
not good for science. CEI: There seems to be an increase in the number of high school teachers who have studied at graduate school. Such teachers have practical experience of research and can tell lively stories of their own experiences to students. RN:
Indeed, it is most important that teachers who have learned the
wonder of science by themselves will share their experiences with
students. One of the reasons why science is not so popular among
young people is that science is treated much too objectively, which
ends in disregard of the people involved. Humans are interested
in humans. Einstein is an overwhelmingly famous scholar. Many people
are enchanted by him not only because of his great scientific achievement
but also because of his unique personality and physical features. CEI: Finally, can you send a message to young people who will read the transcript of this interview? RN: So far scientists have pursued the truth about nature while engineers have solved practical problems faced by society. Hereafter scientists are expected to not only have specific abilities related to their research, but also have an ability to foretell the trends of future society. Both teachers and students should know and understand this point. The crucial point is to create and maintain a sustainable society for our offspring. Science and technology must contribute to this. CEI: Thank you very much for your valuable comments.
Last modified 13.04.07 |
|
| CEI is a newsletter of the Committee on Chemistry Education (CCE) of IUPAC |
 |
 |
 |
 |

 The
Committee on Chemistry Education (CCE) of IUPAC edits and issues
an electronic journal, Chemical Education International (CEI)
(
The
Committee on Chemistry Education (CCE) of IUPAC edits and issues
an electronic journal, Chemical Education International (CEI)
(