|
|
Vol.
30 No. 4
July-August 2008
Mitigating Arsenic Pollution: Bridging the Gap Between Knowledge and Practice*
by Hemda Garelick and Huw Jones
Access to uncontaminated water in sufficient quantities may be the most important requirement for healthy human societies. However, the relationship between the water supply in developing countries and the health of citizens is complex since the relationship is dependent on the provision of both appropriate quantities and quality of water. Attempts to understand this relationship began in the 1970s with the “Bradley Classification” of water-related infection. This was followed by efforts to predict the effect of variations in water quality and supply on morbidity and mortality, particularly of children under five (Cairncross and Valdmanis, 2006). Among the indicators of water quality, the feco-oral (specifically infectious diarrhea) group has historically been important and remains one of the largest water-related contributors to disease on a global scale.
According to some current estimates, approximately 1.8 million people, 90 percent of whom are children under 5, still die every year from diarrheal diseases—mostly in developing countries (WHO, 2008). It is estimated that in industrialized countries, 60 percent of diarrheal disease is attributed to unsafe water, sanitation, and hygiene, whereas in developing countries as much as 85-90 percent of diarrheal illness can be attributed to these causes (Keusch et al., 2006).
While the microbiological quality of water is directly related to human health impacts, access to appropriate quantities of water is an additional health benefit that should not be underestimated. Adequate quantities of water enable people to have improved hygiene, leading to a reduced infectious burden (Cairncross and Valdmanis, 2006). In addition, water availability beyond that required for sustenance and hygiene can confer further health benefits by allowing increased food production in the dry season.
The years 1980–1990 were declared as the U.N. Water Decade. This influenced the work of governments and international agencies in the drive to provide clean and affordable water supplies to all. As part of this drive, international collaborative efforts led by UNICEF resulted in the sinking of millions of tubewells to extract groundwater, which is typically of superior microbiological quality when compared to surface water.
In Bangladesh alone some 10 million tubewells were drilled. UNICEF figures published in 1998 show a 47 percent reduction in infant and under 5 mortality rates in Bangladesh in the years 1980–1996 (UNICEF, 1998). However, many of the tubewells were unknowingly sunk into arsenic-contaminated aquifers. As a consequence, although microbiologically superior water supplies were obtained, some 40 million people subsequently were exposed to toxic levels of arsenic, sometimes exceeding the World Health Organization (WHO) Guideline value by a factor of 20 or more. Of 64 districts within Bangladesh, 59 were reported to contain unsafe levels of arsenic (Caussy and Priest, 2008). The situation in Bangladesh has been described as the biggest case of mass poisoning in recent history.
High levels of arsenic in drinking water have also been found elsewhere in Asia (e.g., Cambodia, China) as well as in the USA and South America. The WHO and U.S. Environmental Protection Agency (EPA) recommended limit for arsenic in drinking water is currently 10 µg/L (WHO, 2006).
There is clearly a pressing need to mitigate the problem. However, it is not so much the difficulty of removing arsenic from water, as it is the extremely low levels to which it must be reduced to ensure safety that presents the challenge to water treatment initiatives, especially in developing countries where the issues of cost and expertise often make “high-tech” solutions impractical. The challenge is not only to provide cheap and efficient treatment but also to develop technologies that are suitable for the large number of small and isolated point sources that are typical of affected rural populations.
The IUPAC Project
By the end of the millennium, the problem of arsenic contamination, particularly in Bangladesh was well documented. The implications to human health from arsenic exposure have been widely investigated by the scientific community. However, from the perspective of those living in affected areas, these problems are not fully understood and solutions for mitigation have not been adequately evaluated or communicated. It is against this background that an IUPAC project (#2003-017-2-600), established under the auspices of the Chemistry and the Environment Division, aims to accomplish the following:
- provide a global overview of natural and anthropogenic sources of arsenic in the water environment and to assess its behavior
- critically review field testing kits intended to provide cheap, quick, on-site measurements so that contamination can be accurately mapped and remediation efforts effectively monitored
- assess the health risk of arsenic contamination, with special reference to technical challenges for optimizing arsenic remediation measures that are acceptable to affected communities
- conduct critical analysis of appropriate methodologies, evaluate their suitability for different situations, and address the transferability of specific technologies that are currently associated with local conditions
For mitigation/remediation action to be taken, the interrelationships and complexities inherent to the processes need to be recognized and understood. To help achieve this, a conceptual model describing these relationships is presented (figure 1). It serves as a framework for the integration of these factors and as a basis for discussion in this article.
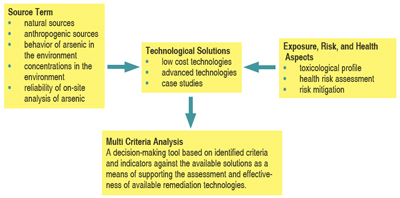 |
| Figure 1: A conceptual model describing the factors and relationship informing the decision-making process for mitigation/remediation of human arsenic exposure. (Garelick et al., 2006) |
Source Term
Distribution
The environmental presence of arsenic derives from both natural and anthropogenic sources. Globally, most arsenic contamination of water occurs naturally when aquifers pass through bedrock containing arsenic. Significant amounts of arsenic are also introduced into the environment from anthropogenic sources, metal mining and smelting being the most important (Garelick et al., 2008).
The main regions of the world affected by arsenic contamination are depicted in figure 2, which also indicates the scale of chronic human exposure. Four principal sources of arsenic are also shown. As can be discerned from the map, areas having natural (geological) contamination of aquifers and regions with mining sources dominate. For coal burning sources, only the Guizhou region of China is shown since this region has the most serious documented problems of this type. Arsenic has also been introduced into the environment through extensive use of arsenical compounds in pesticides. It is also a constituent of wood preservatives (e.g., as copper chromate arsenate). Many sites used by the timber industry to treat lumber have been contaminated with arsenic. Agricultural and/or wood preserving sites of contamination have been reported in South Africa, Zimbabwe, and Australia, but are not included on the map as they are small localized areas.
Analysis and Environmental Sampling
Arsenic’s highly variable distribution in the environment makes it difficult to establish reliable environmental sampling regimes that accurately portray the true status of arsenic contamination. In addition, accomplishing rapid on-site sample analyses at acceptable costs remains a challenge even when reliable sampling has been achieved. A review of these methods is provided by Feldmann (2008). Monitoring for arsenic has historically been centered on drinking water, but more recently monitoring of soil and staple foods has become important because of their potential contribution to human arsenic exposure. On-site analyses of water, soil, sludge, and foodstuffs are essential to ensure that environmental risk assessments properly reflect arsenic residue burdens. Further, those undertaking environmental risk analyses have to consider additional criteria as discussed below.
Exposure, Risk, and Health Aspects
Toxicity
A range of arsenic species is present in the environment, which exhibit complex environmental behavior and large variations in toxicity. The pronounced differences in relative toxicities of six arsenic species are demonstrated in figure 3. The figure, although based on acute studies, further highlights significant differences in toxicity between organic and inorganic species of arsenic. These differences are also supported by a number of field studies, although it is less clear whether these differences are similarly pronounced under conditions of chronic exposure.
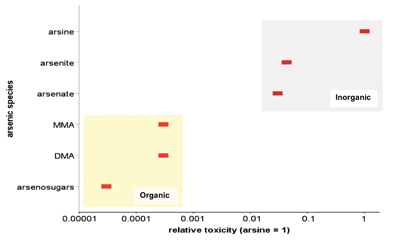 |
| Figure 3: Relative toxicity of arsenic species (MMA = monomethylarsonic acid, DMA = dimethylarsinic acid). Adapted from Vaughan (2006). [Arsine toxicity is based on rat LD50 of 3 mg/kg body weight normalized to 1]. |
Exposure
Humans may be exposed to inorganic arsenic via air (significant intakes by inhalation occur in residents living near industrial sources where exposures to arsenic trioxide are possible), drinking water, food, and soil. However, there are strong indications that consumption of arsenic polluted water is the most important contributor to arsenic-related morbidity. The areas exhibiting the highest levels of human exposure, such as Bangladesh and West Bengal (figure 2), are known to also have the highest occurrence of arsenic contamination of ground water (Caussy and Priest, 2008).
Most food products usually contain less than 250 µg/kg of arsenic. However, seafood, including demersal fish, crustaceans, and marine algae, may contain up to ~100mg/kg of arsenic. The low levels of arsenic in plants contrast with the much higher levels in soil, ~40mg/kg, and may reflect the insolubility of many arsenic-containing minerals such as pyrite under normal soil conditions. The average U.S. daily dietary intake of arsenic is estimated to be 10 to 20µg. In Japan, however, where the seafood content of the diet is high, intakes are much larger (70 to 370µg per day) (Caussy and Priest 2008). The likely predominance of the less toxic organic species of arsenic in foodstuffs may render this source less important in morbidity. However, this is an area that has been less well researched.
Standards and Guidelines
In most countries, the maximum contaminant level (MCL), which is used as an important field monitoring criterion for total arsenic in ground- or potable-water, is set at 50 µg/L. The MCL is either a guideline or an enforceable standard, depending on the country, and is the highest level of a contaminant allowed in drinking water. However, the more stringent WHO guideline value of 10 µg/L has been set as the MCL in recent years. This has now been approved by an increasing number of countries across the world, and has been enforced in the USA since the beginning of 2006. The MCL does not distinguish arsenic species, although as shown in figure 3, speciation is clearly an important factor in arsenic toxicity.
Arsenic levels in soil, sludge, and foodstuffs are less well regulated. Some countries, such as the UK and Canada, have environmental quality criteria for soil (10–50 mg/kg) that are use-dependent. Guidelines for arsenic in foodstuff have not yet been so widely agreed upon. At present, only Australia has established a guideline (1 mg/kg) for total arsenic (no discrimination among species) in foodstuff. China has so far introduced maximum levels for different foodstuffs and was the first country to incorporate arsenic speciation into its guidelines (e.g., 0.15 mg/kg inorganic arsenic for rice). However, the importance of distinguishing among arsenic species in guidelines is an area that requires further progress (Feldmann, 2008).
Risk Considerations
The health outcomes from exposure to arsenic depend not only on the source and chemical species of arsenic, but on dose, modality, and duration of exposure. Both acute and chronic intakes result in toxicity, although drinking contaminated water is only likely to produce effects under conditions of chronic intake. These effects include skin lesions, disturbances of the peripheral nervous system, anemia, and leucopenia, liver damage, circulatory disease, and cancer. Many of these effects have been observed in populations in Taiwan, Argentina, and Bangladesh that consumed contaminated water. The bioavailability of arsenic is a key determinant of the health outcomes of exposure because it is related to the ability of arsenic to be liberated from ingested matrices (e.g., soil, water and food) and thus enter into the blood stream where it exerts its toxic effects. Studies from human volunteer and animal experiments show about 90 percent of ingested, dissolved, inorganic arsenic salts are absorbed from the gut and enter the blood stream. This percentage is much higher than for most other ostensibly non-essential elements and, in part, is a feature of the similar chemical properties of the arsenate (AsO43-) and phosphate (PO43-) ions, the latter being an essential body component that is avidly absorbed from the gut (Caussy and Priest, 2008).
Technological Solutions
Substantial efforts have been invested in developing techniques for removing arsenic from water in both field and laboratory conditions. For any technology to be effective and appropriate for use in affected areas of developing countries, it should use local resources and be low-cost, versatile, transferable, and simple to operate and manage. It is essential that such technologies are accessible to local communities and especially to women. Throughout the developing world, women collect and carry water for their families, use water for cooking and cleaning and for growing food, and therefore should be at the forefront as users of arsenic treatment technologies. Even treatment systems that are highly successful from a technological viewpoint may not succeed in rural areas, such as villages of India and Bangladesh, unless they mesh with local circumstances and are well accepted by the local population.
 |
| Figure 4. Color labelling of safe (green) and unsafe (red) tubewells in Bangladesh. [Photographs courtesy of Feroze Ahmed, Department of Civil Engineering, Bangladesh University of Engineering & Technology, Dhaka, Bangladesh.] |
Although water treatment is central to arsenic pollution mitigation, it is important to recognize that a change in the source of water, rather than treatment of the water locally, may be the most appropriate solution in some cases.
Where municipal piped water supplies exist, connecting to these may provide the appropriate solution for those using contaminated private sources. Even in areas where no municipal piped supplies are provided, switching to a different source well or to harvesting rain water may still be the best solution (Jones et al., 2008). An example of how this may be managed is illustrated by the practice in rural Bangladesh of color-labelling tubewells that provide water deemed unsafe for consumption (figure 4).
Conventional treatment plants may employ several methods for removing arsenic from water. Commonly used processes include oxidation and sedimentation, coagulation and filtration, lime treatment, adsorption onto sorptive media, ion exchange, and membrane filtration. However, in the most affected regions large conventional treatment plants may not be appropriate and factors such as cost and acceptability as well as performance must be considered. A summary of mitigation options, arsenic removal performance, and relative costs are presented in table 1.
|
| Table 1. Evaluation of Performance and Costs of Arsenic Pollution Mitigation Options. Adapted from Visoottiviseth and Ahmed (2008) and Ellis and Garelick (2008). |
Multi Criteria Analysis
The identification and selection of best practices for arsenic mitigation options requires careful evaluation of economic, technological, and environmental factors. Regional social values and inter-disciplinary and inter-institutional contexts are also important considerations. Procedures designed to resolve arsenic remediation must also address complex problems in a manner that is comprehensible to and auditable by stakeholders. Where divergent-minded stakeholders are involved, multi-criteria analysis (MCA) is an approach that can facilitate optimal decision-making.
MCA is a decision-making tool originally developed to help evaluate options that rely on values that are not easily assignable. Cost-benefit analysis is an example where a range of defining criteria (or influencing factors) important to stakeholders are considered. The facilitation of discussions and increased transparency of decision-making processes are possible through MCA since it enables stakeholders to process large amounts of complex information, thereby helping them see the “bigger picture.”
MCA procedures utilize a performance matrix (consequence table) in which options for arsenic remediation are presented (and described) in columns, and performance values for options are presented in rows against predefined criteria. Data may be entered in the matrix as utility scores in various forms, such as numerical, semi-quantitative, and/or descriptive. The performance of each option can then be aggregated against all the criteria from which an eventual overall comparison and decision on the best mitigation option can be made.
Source-exposure vector, health risk, cost, social acceptance, and technical competency are the primary MCA criteria on which a judgement or decision is reached in relation to arsenic treatment options. An example of such a matrix is shown in table 2. The table tracks relevant and appropriate properties for each of the listed criteria that may influence outcomes. Data in the performance matrix is converted into numerical values through application of a specific utility scale scoring and weighting technique for each criterion. A detailed account of these procedures is provided by Ellis and Garelick (2008).
Utility scores and/or weights will clearly influence performance matrix outcomes. Sensitivity analysis can also be performed by reiterating the analysis using different scores or weightings. This is a useful and important approach that enables the MCA methodology to be used as a negotiating tool in the decision-making process, allowing areas of stakeholder agreement and disagreement to be highlighted.
A substantial knowledge pool exists about human exposure to arsenic contamination; the scale of contamination in many regions is well documented, human health effects are known and understood and potential technological solutions have been elucidated. However, the implementation of successful mitigation requires this knowledge to be applied more effectively. This can only be achieved by enhancing the understanding of the interactions between socio-economic and scientific issues, thus enabling practitioners to develop effective and appropriate mitigation programs.
References
Cairncross, S. and Valdmanis, V. (2006) “Water Supply, Sanitation, and Hygiene Promotion.” In Jamison et al. (eds): Disease Control Priorities in Developing Countries (2nd Edition),ed. , 371-388. New York: Oxford University Press. <www.dcp2.org/pubs/DCP/41/>.
Caussy, H. and Priest, N. (2008) “Introduction to Arsenic Contamination and Health Risk Assessment with Special Reference to Bangladesh.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
Ellis, J.B. and Garelick, H. (2008) “A Multi-Criteria Approach for Assessing Options to Remediate Arsenic in Drinking Water.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
Feldmann, J. (2008). “On Site Testing for Arsenic: Field Test Kits.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
Garelick, H. et al. (2006). “Remediation Technologies for the Removal of Arsenic from Water and Wastewater.” An IUPAC project presented at the IUPAC 43rd General Assembly, Beijing, China.
Garelick, H. et al. (2008). “Arsenic Pollution Sources.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
Jones, H. et al. (2008). “Case Report: Arsenic Pollution in Thailand, Bangladesh and Hungary.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
Keusch, G.T. et al. (2006). “Diarrheal Diseases.” In Jamison et al (eds): Disease Control Priorities in Developing Countries (2nd Edition), ed. , 371–388. New York: Oxford University Press. <www.dcp2.org/pubs/DCP>; Chapter 19.
UNICEF (1998) The State of the World’s Children 1998.
<www.unicef.org/sowc98>
Vaughan, D.J. (2006) “Arsenic” Elements 2: 71–75.
Visoottiviseth, P. and Ahmed, F. (2008) “Technology for Remediation and Disposal of Arsenic.” Reviews of Environmental Contamination and Toxicology. 197. [In Press].
WHO (2006) Guidelines for Drinking-Water Quality. Volume 1—Recommendations. First addendum to third addition. <www.who.int/water_sanitation_health/dwq/gdwq0506.pdf>
WHO (2008) “Water Quality Interventions to Prevent Diarrhoea: Cost and Cost-Effectiveness” (T.F. Clasen, L. Haller) Geneva 2008 WHO/HSE/WSH/08.02
*This article arose from work carried out by an IUPAC project (2003-017-2-600) sponsored by the Chemistry and the Environment Division (VI). The participants were F. Ahmed, G. Borbély, M.K. Bux, D.H. Caussy, A. Dybowska, J.B. Ellis, J. Feldmann, R. Földényi, Z. Galbács, H. Garelick, H. Jones, N. Kováts, N. Priest, Y. Shevah, E. Valsami-Jones, and P. Visoottiviseth.
Hemda Garelick <[email protected]> is a professor in the School of Health and Social Sciences at Middlesex University, The Burroughs, London, United Kingdom. Huw Jones <[email protected]> is a professor in the Department of Natural Sciences at Middlesex University.
|
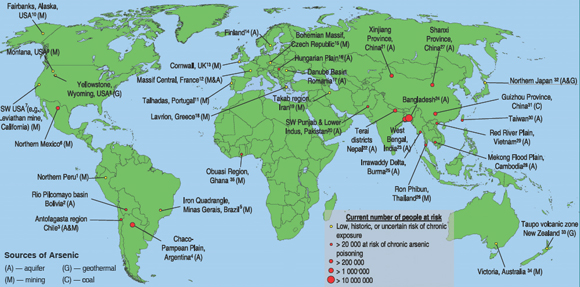 |
| Figure
2. Worldwide distribution of arsenic contaminated regions,
showing source of arsenic and numbers of people at risk
of chronic exposure. Note: estimates of people at risk
of poisoning are very difficult to quantify, particularly
in areas where geochemical surveys are limited. These
estimates are broad and are based on four criteria: 1)
prevalence of current recorded cases of arsenicosis, 2)
likelihood of ingested concentrations exceeding 50 µg/L)
number of people living in exposed areas, 4) likely ability
of region to mitigate/remediate against contamination.
[View
larger image.] |
Map References
1. Bech J, Poschenrieder C, Llugany M , Barceló J , Tume P, Tobias FJ, Barranzuela JL & Vásquez ER (1997). Arsenic and heavy metal contamination of soil and vegetation around a copper mine in Northern Peru. Sci. Total Environ. 203(1): 83-91.
2. Archer J, Hudson-Edwards KA, Preston DA, Howarth RJ & Linge K (2005). Aqueous exposure and uptake of arsenic by riverside communities affected by mining contamination in the Río Pilcomayo basin, Bolivia. Mineral. Mag. 69(5):719-736.
3. Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C & Gibb H. (2000). Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 108(7):667-673.
4. Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, Bonorino G & Albouy R. (2004). Groundwater arsenic in the Chaco-Pampean Plain, Argentina - case study from Robles county, Santiago del Estero Province. Appl. Geochem. 19:231-243
5. Matschullat J, Birmann K, Borba RP, Ciminelli V, Deschamps EM, Figueiredo BR, Gabrio T, Haßler S, Hilscher A, Junghänel I, de Oliveira N, Raßbach K, Schmidt H, Schwenk M, de Oliveira Vilhena MJ & Weidne U. (2007). Long-term environmental impact of arsenic-dispersion in Minas Gerais, Brazil. Trace Metals and other Contaminants in the Environment. 9:365-382.
6. Cebrian ME, Albores A, Aguilar M & Blakely E. (1983). Arsenic Poisoning in the North of Mexico. Human Toxicology 2 121-133.
7. Webster JG, Nordstrom DK & Smith KS. (1994). Transport and natural attenuation of Cu Zn As and Fe in the acid mine drainage of Leviathan and Bryant Creeks. In: Alpers CN, Blowes DW (Eds) Environmental Geochemistry of Sulphide Oxidation. Am Chem Soc Symp Ser 550 ACS Washington DC 244-260.
8. Webster JG & Nordstrom DK (2003). Geothermal arsenic. In: Welch AH, Stollenwerk KG, (Eds) Arsenic in Ground Water: Geochemistry and Occurrence, Kluwer Academic Publishers Boston, pp101-126.
9. Moore JN & Woessner WW (2003). Arsenic contamination in the water supply of Milltown Montana. In: Welch AH, Stollenwerk KG (Eds) Arsenic in Ground Water: Geochemistry and Occurrence, Kluwer Academic Publishers, Norwell, Massachusetts pp 329-350.
10. Wilson FH & Hawkins DB (1978). Arsenic in streams stream sediments and ground water, Fairbanks area, Alaska. Environ Geol 2:195-202.
11. Patinha C, Da Silva EF & Fonseca EC, (2004). Mobilisation of arsenic at the Talhadas old mining area - Central Portugal. J Geochem Explor 84(3):167-180.
12. Criaud A & Fouillac C (1989). The distribution of arsenic(III) and arsenic(V) in geothermal waters - Examples from the Massif Central of France the Island of Dominica in the Leeward Islands of the Caribbean the Valles Caldera of New-Mexico United-States and Southwest Bulgaria. Chem Geol 76(3-4):259-269.
13. Thornton I & Farago ME (1997). The geochemistry of arsenic. In: Abernathy CO, Calderon RL, Chappell WR (Eds) Arsenic Exposure and Health Effects. Chapman & Hall London pp 1-16.
14. Kurttio P, Pukkala E, Kahelin H, Auvinen A & Pekkanen J. (1999). Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect 107(9):705-710.
15. Filippi M, Golias V & Pertold Z (2004). Arsenic in contaminated soils and anthropogenic deposits at the Mokrsko Roudny´ and Kas¡perske´ Hory gold deposits Bohemian Massif (CZ). Environ Geol 45:716-730.
16. Földényi, R , Kováts, N, Borbély, G & Galbács, Z (2008). Arsenic in drinking water supply in Hungary. Rev. Environ. Contam. Toxicol. 197 (in print)
17. Gurzau ES & Gurzay AE. (2001). Arsenic in drinking water from groundwater in Transylvania, Romania 181-184. In Chappell WR Abernathy CO, Calderon RL, (Eds) Arsenic Exposure and Health Effects IV. Elsevier, Amsterdam.
18. Komnitsas K, Xenidis A & Adam K (1995). Oxidation of pyrite and arsenopyrite in sulphidic spoils in Lavrion. Miner. Eng. 12:1443-1454.
19. Modabberi S & Moore F (2004). Environmental geochemistry of Zarshuran Au-As deposit NW Iran. Envirol Geol 46:796-807.
20. Shrestha B. (2002). Drinking water quality: future directions for UNICEF in Pakistan. Consultancy report 2 of 3, Water Quality, SWEET Project, UNICEF Pakistan, Islamabad.
21. Wang GQ, Huang YZ, Gang JM, Wang SZ, Xiao BY, Yao H, Hu Y, Gu YL, Zhang C & Liu KT (2000). Endemic arsenism fluorosis and arsenic-fluoride poisoning caused by drinking water in Kuitun, Xinjiang. Chin Med J 113:524.
22. Shrestha RR, Shrestha MP, Upadhyay NP, Pradhan R, Khadka R, Maskey A, Maharjan M, Tuladhar S, Dahal BM & Shrestha K. (2003). Groundwater Arsenic Contamination, Its Health Impact and Mitigation Program in Nepal. J Environ Sci Health, Part A. 38(1):185- 200.
23. Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G & Chakraborti D (1995). Arsenic in groundwater in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part I: Arsenic species in drinking water and urine of the affected people. Analyst 120:643-650.
24. Ahmad K (2001). Report highlights widespread arsenic contamination in Bangladesh. The Lancet 358:133
25. Tun TN (2003). Arsenic contaminated water sources in rural Myanmar. Proceedings of the 29th WEDC International Conference "Towards the Millenium Development Goals". pp219 -221. Abuja, Nigeria.
26. Williams M, Fordyce F, Paijitprapapon A, Charoenchaisri P (1996). Arsenic contamination in surface drainage and groundwater in part of the southeast Asian tin belt, Nakhon Si Thammarat Province, southern Thailand. Environ Geol 27:16-33.
27. Xia Y, Liu J. (2004). An overview on chronic arsenism via drinking water in PR China. Toxicology 198 (1-3) 25-29.
28. Berg M, Stengel C, Trang PTK, Viet PH, Sampson ML, Leng M, Samreth S & Frederick, D. (2007). Magnitude of arsenic pollution in the Mekong and Red River Deltas - Cambodia and Vietnam. Sci. Tot. Env. 372: 413-425.
29. Berg M, Tran HC, Nguyen TC, Pham HV, Schertenleib R & Geiger W (2001). Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ Sci Technol 35(13):2621-2626.
30. Hsu KH, Froines JR, Chen CH (1997). Studies of arsenic ingestion from drinking water in Northestern Taiwan: chemical speciation and urinary metabolites. In: Abernathy CO, Calderon RL, Chappell WR (Eds) Arsenic Exposure and Health Effects Chapman & Hall, London. pp190-209.
31. Dai S, Ren D, Tang Y, Yue M & Hao L (2005). Concentration and distribution of elements in Late Permian coals from western Guizhou Province China International. J Coal Geol 61(1-2):119-137.
32. Masuda H, Ibuki Y & Tonokai K. (1999). Mechanism of natural arsenic pollution of shallow groundwater in the northern part of Osaka prefecture, Japan, J. Groundwater Hydrol 41(3):133-146.
33. Webster JG & Nordstrom DK (2003). Geothermal arsenic. In: Welch AH, Stollenwerk KG, (Eds) Arsenic in Ground Water: Geochemistry and Occurrence, Kluwer Academic Publishers Boston, pp101-126.
34. Smith E, Smith J, Smith L, Biswas T, Correll R & Naidu R. (2003). Arsenic in Australian environment: an overview. J Environl Sci Health, Part A: Toxic/Hazardous Substances and Environmental Engineering, A38 (1): 223-240.
35. Amonoo-Neizer EH & Amekoe EMK. (1993). Determination of total arsenic in environmental samples from Kumasi and Obuasi, Ghana. Environ Health Perspect 101(1): 44-49. |
| |
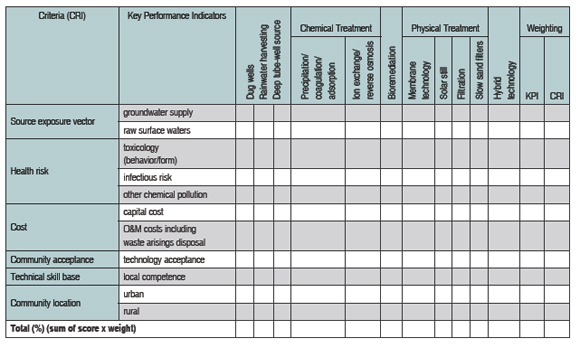 |
| Table 2 provides an example of the MCA procedure in which a performance matrix is constructed from
utility scores for each key performance indicator. [View full-page pdf.] |
Page last modified 5 August 2008.
Copyright © 2003-2008 International Union of Pure and Applied Chemistry.
Questions regarding the website, please contact [email protected] |