|
|
Vol.
34 No. 4
July-August 2012
|
| |
Recent IUPAC technical reports and recommendations that affect the many fields of pure and applied chemistry.
See also www.iupac.org/publications/pac |
F. Arico, A. Vavasori, Z. Liu, and T. Jiang (eds.)
Special Topic Issue, Pure and Applied Chemistry, 2012, Vol. 84, No. 3, pp. 411–860
A special topic issue of Pure and Applied Chemistry published in March 2012 and titled "Chlorine-free synthesis for Green Chemistry" explores the restriction, or preferably prevention, of the use of halogenated compounds, whenever feasible, through the assembly and reporting of already identified information. Innovative synthetic pathways using clearly identified production drivers (e.g., environmental and health impact, energy consumption, economical feasibility, etc.) have been elucidated. In past decades, scientific knowledge and feasible technologies were unavailable, but now there is enough expertise to pursue discontinuation of hazardous and toxic reagents. This PAC Special Topic issue presents a collection of useful and industrially relevant examples for alternatives to chlorine in synthesis.
by Pietro Tundo
In the last 20 years, chemists have put enormous effort into designing chemicals with various applications ranging from medicines and cosmetics to materials and molecular machines. However, for the most part, their work demonstrated a quite surprising lack of interest in taking hazards into consideration in the design process. The goal was often to design substances that were robust and could last as long as possible. This philosophy has resulted in a legacy of persistent toxic and bio-accumulative substances and lingering toxic waste sites. Nowadays, it is known that it is more desirable to avoid substances that persist indefinitely in the environment or in a landfill, and to replace them with substances designed to degrade after use. Polymeric materials, for instance, should have no negative effect on the environment during their production, utilization, or disposal. Therefore, the design of safer chemicals cannot be limited to hazards associated with the manufacture and use of the chemical, but also to its disposal (i.e., its full life cycle).
Among halogens, chlorine is by far the most abundant chemical in nature and also the easiest to produce and use. This explains its predominant and seemingly irreplaceable role in the chemical industry (see figure). Five hundred companies at 650 sites around the world have the capacity to produce over 58 x 106 tonnes of chlorine and 62 x 106 tonnes of its co-product, caustic soda, per year.
For example, the European chloro-alkali industry had a production in 2009 of 9.1 x 106 tonnes at about 80 plants, mostly (about 95 percent) via electrolysis-based techniques (chlor-alkali industry); the sector directly employs about 40 000 people in 20 European Union countries. Germany is Europe's largest chlorine producer, accounting in 2009 for 43.5 percent of European production.1 Owing to their peculiar characteristics, halogens are widely used by all sectors of the chemical industry to produce solvents, catalysts, building blocks, additives, and drugs. Chlorine is a major building block in today's chemistry. More than 90 percent of pharmaceuticals contain or are manufactured using chlorine, which is also used in the production of 86 percent of crop protection chemicals. Furthermore, halogens are contained in several commodities that we all use daily as plastics (e.g., chlorine is contained in polyvinyl chloride, PVC, one of the most widely used plastic materials), solvents for dry cleaning and metal degreasing, textiles, agrochemicals and pharmaceuticals, insecticides, dyestuffs, household cleaning products, and disinfectants. Chlorine is used extensively in organic and inorganic chemistry as an oxidizing agent (i.e., water disinfectant) and as a leaving group in substitution and elimination reactions.
Chlorine compounds find use as intermediates in the production of a number of important commercial products that do not contain chlorine. Foremost examples are polycarbonates, polyurethanes, silicones, polytetrafluoroethylene, carboxymethyl cellulose, and propylene oxide.
Through a chain of chemical derivatives and relatively easily made compounds and intermediates, such molecules have used the intrinsic energy available through the use of chlorine primarily produced via electrolysis.
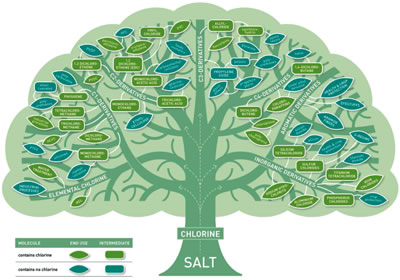 |
| Chlorine tree showing most of the derivatives and applications of chlorine chemistry. |
The widespread use of halocarbons was often driven by observations that most of them were more stable than other substances. Their stability tended to encourage beliefs that they were mostly harmless, but starting from the mid-1920s it was discovered that they can cause chloracne or fatal liver diseases in workers in the chemical industries. By the 1950s, toxicity and health hazards related to halocarbons were widely reported. Concerns about the environmental and health impact of halocarbons were first raised early in the 1960s in studies about DDT and other halocarbon pesticides. Today, they are widely recognized as persistent pollutants and doubts were recently (2006) raised even about very stable molecules such as perfluorooctanoic acid (PFOA) and Teflon, just to mention a few.
Today, European and international legislation for environmental protection is becoming stricter and recognizes that there is a growing need for replacement of halogenated compounds at a productive and end-user level. Besides EU directives, which tackle global environmental concerns (e.g., sustainable development) through prescription about chemical production in the frame of a multi-sector approach (political, scientific, economical, and social), some regulations are specific to ban or restrict the production of some chemical compounds or byproducts at an industrial level.
The EU has recently established REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), a single integrated system for the registration, evaluation, and authorization of chemicals, together with the European Chemicals Agency (ECHA). REACH requires firms that manufacture and import chemicals to evaluate the risks resulting from the use of those chemicals and to take the necessary steps to manage any identified risk. Industry has the burden of proving that chemicals placed on the market are safe. By the end of 2008, ECHA received approximately 2.75 million requests for preliminary authorization concerning about 150 000 compounds collected in an ECHA online database. Among them, over 20 000 substances are chlorinated and more than 4000 are brominated (this excludes those where "chlorine is used in the making", which would increase these numbers considerably). The process for authorization will be long (possibly ending in 2018), but a significant fraction of halogenated substances are at risk of rejection, thus forcing their replacement at a productive level.
Furthermore, some international conventions ban or restrict production of specific chemicals. At least two need to be mentioned. The Stockholm Convention on Persistent Organic Pollutants (most of which contain chlorine; byproducts of both domestic and industrial activities) and the Montreal Protocol on Substances that Deplete the Ozone Layer which control the phase-out of ozone depleting substance (ODS) production and use. The application of both is managed by the United Nations Environmental Program, and all their prescriptions will come into force within 2020. Finally, we must cite the Kyoto Protocol about greenhouse gas (GHG) emissions, which was adopted almost worldwide and then mostly disregarded. International agreements about a follow-up to the Kyoto Protocol are currently in progress.
Today, the European and international legislation and EU directives for environmental protection are becoming stricter and recognize that there is a growing need for replacement of particular halogenated compounds at a productive and end-user level. In 2007, the Intergovernmental Panel on Climate Change stated that some halocarbons were a direct cause of global warming, owing to their nature as GHGs. Some of them are ODSs. Halogen-based materials may show an indirect toxicity or eco-toxicity, releasing halogen atoms/molecules and/or harmful compounds (e.g., in case of accidental fires, a case of particular interest for the electric distribution industry).
Since the industrial revolution, the halogen chlorine remains "an iconic molecule" for industrial chemical production. Even though its production by the electrolysis of sodium chloride is very energy-intensive, it is still used because it allows the manufacture of chlorinated derivatives in a very easy way, owing to its high energy and reactivity; for example, easily obtained from chlorine are AlCl3, SnCl4, TiCl4, SiCl4, ZnCl2, PCl3, PCl5, POCl3, COCl2, etc., which in turn are pillar intermediates in the production of numerous everyday goods. This kind of chloride chemistry is widely utilized because the energy is transferred to these intermediates, making further syntheses easy; a good number of fundamental reactions of the industrial production are based on the synthesis of chloride compounds obtained by reaction with SOCl2, COCl2, or AlCl3 as a catalyst.
Besides their (eco-) toxicity, a major concern with chlorine derivatives is the large amount of energy necessary for their production, and this is why chlorinated molecules have both a direct (as GHGs) and indirect (CO2 production) impact on climate change at a global level.
Estimates of the global warming potential (GWP) resulting from chlorine use by the chlorine industry in Europe is 0.29 percent of the total GWP, while the estimate from the primary energy consumed is 0.45 percent of the total GWP.
The chlorine industry is extremely energy-intensive: its CO2 emissions is comparable to that of the iron and steel industry and higher than for cement (1.5 kg CO2/kg for the chlorine industry vs. 1.7 for iron and steel and, 0.95 for cement) and that for a world production of the order of 40 x 106 tonnes (covering Europe and China in 2008) vs. 1.2 x 109 tones of iron and steel, and 2.3 x 109 tonnes of cement.2
Can we pursue an intrinsically safer, cleaner, and more energy-efficient alternative to chlorine chemistry? Many of society's greatest challenges and fortunes depend on the development of the chloro-alkali industry; but is chlorine-based chemistry sustainable?
Chlorine-based chemistry very often does not obey the principles of atom economy and waste minimization introduced, respectively, by B. Trost3 and R. Sheldon4; in fact, halogen anions are byproducts of many organic reactions and represent a waste to be disposed of. The environmental and health constraints (toxicity and eco-toxicity, ozone layer depletion) and the growing need for energy (energy efficiency, climate change) force us to take advantage of available knowledge to develop a new chemical strategy. By the motivation given, it seems appropriate to refer to chlorine-free chemistry as "beyond-chlorine chemistry."
The beyond-chlorine strategy has two approaches, bottom-up and top-down, as follows:
- The bottom-up approach involves investigating halogen-free reactions and processes on a lab scale in academies and then scaling them up for production by industry (the most appropriate term would be green chemistry).
- The top-down approach involves industry collaboration with academic partners to design halogen-free alternatives for industrial products and processes (the most appropriate term would be sustainable chemistry).
Beyond-chloro does not mean, of course, that we should avoid chloride anion, for example in foods or as disinfectant. The substitution of chlorine compounds and of compounds where "chlorine is used in the making" means that we avoid electrolysis as the primary energetic source; however, this makes chemistry "without chlorine" considerably more difficult and illustrates why it has not been adopted before.
The rationale behind this Special Topic issue is to seek useful and industrially relevant examples for alternatives to chlorine in synthesis, so as to facilitate the development of industrially relevant and implementable breakthrough technologies. The 29 papers included in the issue are partitioned into the following parts:
- Chlorine-Free Reagents And Reaction Selectivity
- Chlorine-Free Catalysts
- Carbonate Chemistry
- Chlorine-Free Solvents
- Benign Chloro-Free Methodologies
- Metrics On Chlorine-Free Syntheses
www.iupac.org/publications/pac/84/3/
- Chlorine Industry Review 2009–2010, Eurochlor Magazine <www.eurochlor.org>.
- K.-O. Feldmann, S. Schulz, F. Klotter, J.J. Weigend. ChemSusChem 4, 1805 (2011).
- B.M. Trost. Science 254, 1471 (1991).
- R.A. Sheldon. Chem. Ind. (London) 12 (1997).
Department of Environmental Sciences, Ca' Foscari University of Venice, Venice, Italy; E-mail:
[email protected] ; reproduced in part from PAC 84(3), pp. 411–423, 2012; http://dx.doi.org/10.1351/PAC-CON-12-02-02.
Page
last modified 10 July 2012.
Copyright © 2003-2012 International Union of Pure and Applied Chemistry.
Questions regarding the website, please contact [email protected] |