Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
F
Fermi hole - A region around an electron
where the probability of finding another electron with the same
spin is very small due to the action of the antisymmetry
principle.The Fermi hole describes the manner in which the
charge of the reference electron is spread out in space, thereby
excluding the presence of an identical amount of same-spin
density. McWEENY (1960).
Fermi level - In an extended structure
(solids, metals, semiconductors or insulators), the average for the
highest occupied and the lowest unoccupied levels. In chemistry, the
Fermi level is usually considered as the highest occupied
crystal orbital.
Fluxional molecules - A subclass
of structurally nonrigid
molecules in which all the interconverting species that are
observable are chemically and structurally equivalent. The classic example
of the phenomenon of fluxionality is the rapid automerization
of tricyclo[3.3.2.02,8]deca-3,6,9-triene (bullvalene), the
rapid interconversion of 1 209 600 (10!/3) degenerate isomers. COTTON
(1975).
Force field - Within the molecular
mechanics approach, a set of potential functions defining bond
stretch, bond angle (both valence and dihedral) distortion energy
of a molecule as compared with its nonstrained conformation (that
characterized by standard values of bond
lengths and angles). A set of transferable empirical force
constants is preassigned and the
harmonic approximation is usually employed. Some force
fields may contain terms for interactions between nonbonded atoms,
electrostatic, hydrogen bond and other structural effects as well as
account for inharmonicity effects.
In vibrational spectroscopy, the inverse problem is solved by determining
a set of force constants and other parameters of a chosen potential
energy function which would match with experimentally observed vibrational
frequencies of a given series of congeneric molecules.
Fractional bond number (synonymous
with the Pauling's bond order)
- The formal bond order, n defined in terms of
the difference between a standard single bond distance r0
and an interatomic distance r, and an empirical constant c.
c ln n = r0 - r
Fragment molecular orbital analysis
- (Synonymous with reconstruction molecular orbital
analysis) - The procedure based on the theory of orbital
interactions for the construction of molecular
orbitals of a complex molecular system from the orbitals
of its fragments with the same geometry and symmetry properties
as these fragments have in the molecule. HOFFMANN,
ALBRIGHT, and THORN (1981).
Franck-Condon principle -
Classically, the approximation that an electronic transition is
most likely to occur without changes in the positions of the nuclei
in a molecular entity and
its environment. The resulting state is called a Franck-Condon
state, and the transition involved is called a vertical
transition. The quantum mechanical formulation of this principle is
that the intensity of a vibronic
transition is proportional to the square of the overlap
integral between the vibrational wavefunctions of the two
states involved in the transition. IUPAC
PHOTOCHEMICAL GLOSSARY (1988).
Frank-Condon State - see Frank-Condon
principle.
Free radical - see Radical.
Free valence - A reactivity index
applied mostly to radical reactions of conjugated systems. An atom in
such a system can exert only a certain maximum of
p-bonding power as measured by a sum of the p- bond
orders PAB of bonds formed by it. In the Hückel
molecular orbital theory, the maximum p-bonding
power for a carbon atom is ˜3;
hence the free valence of a carbon atom in a molecule is defined
by
FA = 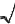 3
-
3
- 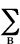 PAB
PAB
Frontier function (synonymous
with Fukui function) - The
function which provides a description of electron
density in the frontier
orbital as the initial response in the
electron density at position r, r(r),
due to infinitesmal perturbation in the total number of electrons N
in the molecular entity.
f(r) = � r(r)/�
N
FUKUI (1982); REED
(1994).
Frontier orbitals - The highest
occupied molecular orbital (HOMO) and the lowest
unoccupied molecular orbital (LUMO) of a given molecular
entity. In the case of an odd-electron molecular entity, when its
HOMO is occupied by a single electron such a molecular orbital is
termed a singly occupied molecular
orbital (SOMO). Depending on the properties of the reactive
partner, the SOMO of a given species may function as either HOMO or
LUMO. The special importance of the frontier orbitals is due to
the fact that a broad variety of chemical reactions takes place
at a position and in a direction where the overlap of HOMO and LUMO
of the respective reactants is maximal.
Frozen core approximation - Approximation
commonly used in post-SCF calculations which implies that only
the contributions of the valence electrons to the correlation energy
are evaluated.
Fukui function - see Frontier
function

